Background: Light chain (AL) amyloidosis is a rare plasma cell disorder (PCD) characterized by misfolded light chains produced by clonal plasma cells leading to organ dysfunction. Over the past few years, the introduction of daratumumab (dara), a CD38 monoclonal antibody, into clinical practice has markedly improved outcomes, however, relapses still occur with limited available options. Venetoclax (VEN) is a BCL-2 inhibitor that has shown encouraging clinical efficacy in PCDs harboring the translocation between the chromosomes 11 and 14 (t[11;14]). The aim of this study was to evaluate the efficacy of VEN-based therapy (VBT) in patients with dara-refractory AL who were positive for t(11;14).
Methods:Of all adult patients with systemic AL amyloidosis evaluated at our center between 1/2016 and 7/2023, we focused on those who had prior refractoriness to dara (dara was given at any time during the disease course, as frontline or salvage treatment) and received VBT (at any time after dara refractoriness). Data was obtained via retrospective chart review. Hematologic responses including complete response (CR), very good partial response (VGPR) and partial response (PR) were according to the AL consensus criteria. No patients had concomitant multiple myeloma. Baseline characteristics were outlined by descriptive analysis. Kaplan-Meier method was used for progression free survival (PFS), time-to-next-treatment (TTNT) and overall survival (OS) calculations.
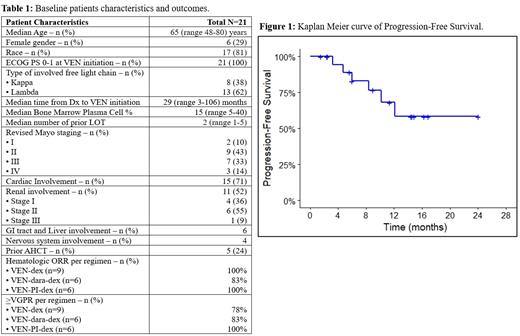
Results: A total of 21 patients who met the above criteria, were included in this analysis. The median age of patients was 65 (range 48-80) years, median number of prior lines of therapy (LOT) was 2 (range 1-5) and median percentage of bone marrow plasma cells prior to VBT initiation was 15 (range 5-40). Fifteen (71%) patients had cardiac involvement, 11 (52%) renal involvement, and 7 (33%) in addition to other organs had bone marrow involvement; 67% of patients had >2 organs involved. Of the entire cohort, 24% had undergone autologous hematopoietic cell transplant (AHCT) as frontline therapy. All patients had t(11;14) and one patient had an additional 1q gain. The baseline characteristics are summarized in Table 1. VEN was given in combination with: dexamethasone (VEN-dex) in 9 patients (43%), daratumumab-dex (VEN-dara-dex) in 6 patients (28.5%) and proteasome inhibitor-dex (VEN-PI-dex) in 6 patients (28.5%). The median duration on VBT was 9 (range 1.5-24.1) months. At data cut-off, 5 patients had discontinued therapy because of ongoing CR/VGPR, 1 due to ongoing fatigue and 6 due to disease progression or death. The hematologic overall response rate (ORR) was 95% with 11 (53%) patients achieving CR or better and 7 achieving (33%) VGPR. Patients responded rapidly, with a median time to best hematologic response being 2 (range1-5) months. Among the 18 (86%) patients who achieved >VGPR, 15 also achieved difference of free light chains (dFLC) <10 mg/l. Of the 15 patients with cardiac involvement, 6 (40%) achieved response to VBT, while 4 (27%) were non-evaluable (3 had maintained response from previous LOT and 1 had undergone heart transplant); the remaining 5 (33%) had stable disease without response. Of the 11 patients with renal involvement, 4 (36%) achieved organ response to VBT, while 2 (18%) were non-evaluable (1 had maintained response from previous LOT and 1 had undergone kidney transplant); the remaining 5 (46%) had stable disease without response. Eventually, 4/21 patients experienced disease progression. The median PFS for the entire cohort was not-reached (NR); the 6 and 12-month PFS rates were 83% and 68% respectively. Median TTNT was also NR. At data cut-off, 3 patients had died (2 from cardiac complications [cardiac arrest and heart failure], and 1 from dialysis complications). The median OS was NR; the 6- and 12-month OS rates were 89% and 83%, respectively.
Conclusion: Our findings suggest that VEN is effective in t(11;14) positive relapsed-refractory AL amyloidosis in combination with dexamethasone alone or with other established plasma-cell directed agents. VBT led to deep and rapid overall response rates with more than 80% of patients achieving VGPR after exposure to only 2 cycles of therapy. This is critical in the setting of dara-refractoriness where therapeutic options are very limited. A clinical trial is ongoing to further evaluate the role of VEN in relapsed-refractory AL amyloidosis.
Disclosures
Williams:Abbvie: Consultancy; Janssen: Consultancy; Bristol Meyers Squibb: Consultancy. Valent:Alexion, AstraZeneca Rare Disease: Research Funding. Raza:Prothena: Honoraria; Pfizer: Honoraria; Kite Pharma: Honoraria; ATARA Therapeutics: Current holder of stock options in a privately-held company; Autolus Therapeutics: Current holder of stock options in a privately-held company. Khouri:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; GPCR Therapeutics: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events.